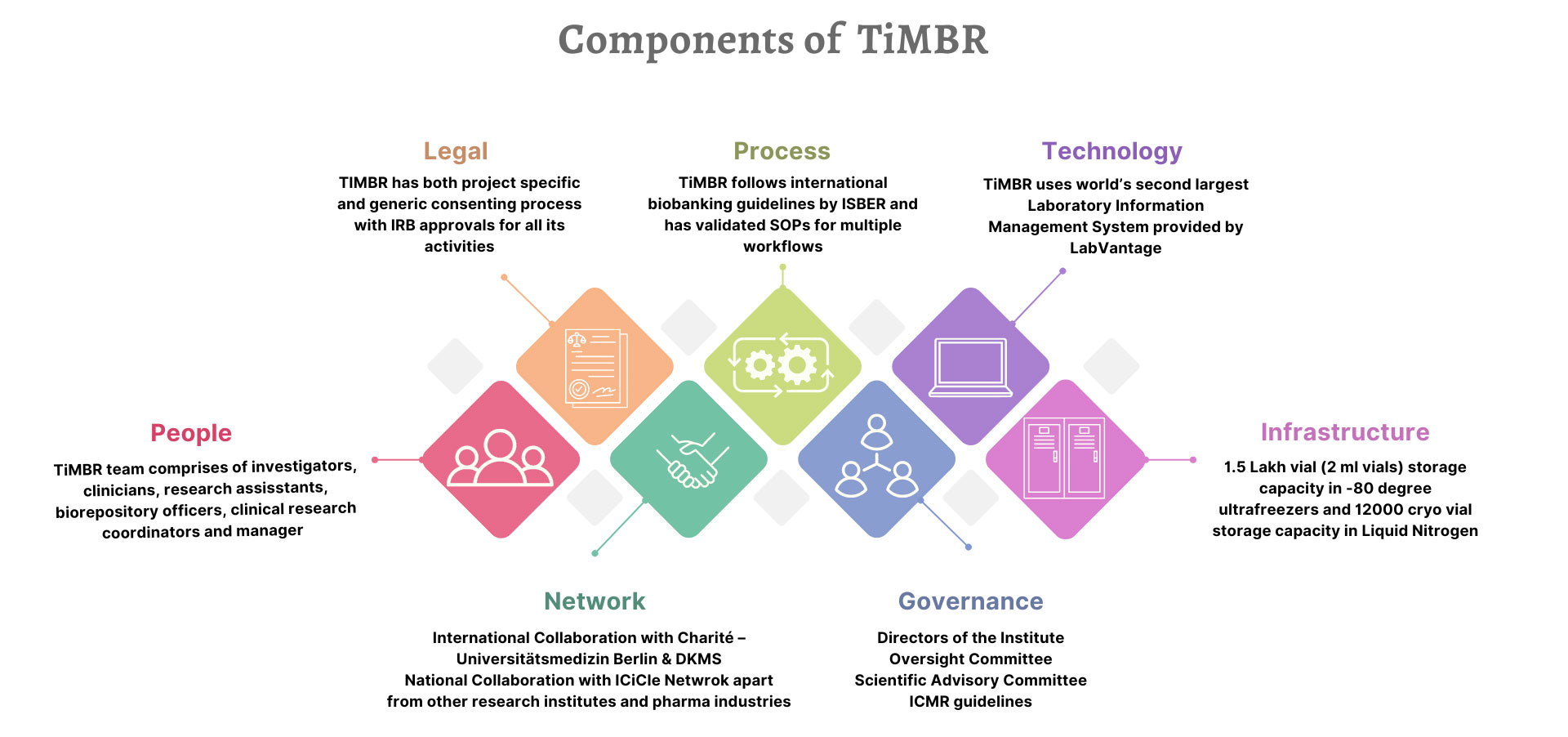
Biorepository Capacity and Tracking
The current overall vial storage capacity of TiMBR is up to 1.5 lakhs tube samples. The biorepository is equipped to process and store whole blood, buffy coats, sera, plasma, cerebrospinal fluid, DNA, RNA and tissue. All biospecimens are tracked throughout their life cycle—from collection to sample disposition—using a customized laboratory information system - LabVantage.
Plan
- Objective of the project
- Inclusion and exclusion criteria
- Study Design
- Timeline
- Excess Sample Utilisation
Regulate
- Approvals from Institutional Review Board & TiMBR Oversight Committee
- Ethical Documents
- Funding and other resources
- Standard Operating Protocols SOP
- Data Management
Collect
- Consent
- Sample in predefined conditions as per downstream requirement-SOP
- Clinical Information
Transport
- Samples in predefined conditions to maintain integrity till processing-SOP
- Pseudo-anonymized Data (Hospital Management System to LabVantage)
Process
- Samples following SOPs based on sample type and downstream activity
- Sample information and relevant clinical information
Protect
- Samples in predefined storage conditions as per downstream requirement
- Storage Units– Daily manual vigilance and remote monitoring
- Prompt breakdown reporting and back up actions
- Sample information through LIMS
Retrieve
- Sample availability and location information from LabVantage
- Samples following SOPs based on sample type and downstream activity
Analyse
- Analyse samples for biomarker identification, drug response and efficacy
Repurpose-Dispose
- Repurpose unutilised samples for other activities (eg.: Quality control)
- Dispose excess unutilisable samples as per discard policy
Report
- Clinically relevant information
- Present to stakeholders
- Publish